April 20, 2018
The availability and waiting time for reimbursed drugs with orphan status
HTA Consulting examined the availability and waiting time for reimbursed drugs with orphan status registered by EMA in Poland in 2004-2018 (18/04/2018).
Currently in Poland (as of 18/04/2018), 21 innovative medicines with orphan status (out of 104) registered in EMA are reimbursed. 8 of them were registered after 2012.01.01.
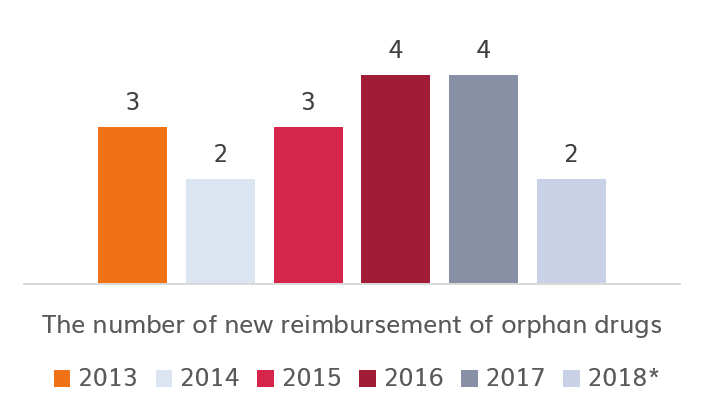
Source: own study | * Current on day of 2018.03.01
The average waiting time for reimbursement of treatment with orphan drug status was in days:
| Authorisation date by EMA | Time [days] |
| Since 2006 | 1679* (about 4,5 years) |
| After 2012 | 938 (about 2,5 years) |
* Excluding drugs reimbursed before 2012
- The total cost of reimbursement of the analyzed drugs in 2017 amounted to approx. PLN 361 million (of which PLN 135 million is Revlimid).
- 14 of the analyzed reimbursed drugs are in oncological indications, while the remaining ones in non-oncological indications.
Drugs with orphan status reimbursed in Poland (click to enlarge):
Source: own study based on IKAR pro data



